National Taiwan University research team uses single-cell analysis to reveal how exhausted T cells drive immunotherapy resistance and identify a potential new therapeutic strategy.
Immune checkpoint inhibitors (ICIs) have revolutionized cancer therapy by harnessing the body’s immune system to target tumors. Despite their initial clinical success, many patients eventually experience diminished therapeutic responses as tumors acquire resistance. A new study from Taiwan now elucidates the underlying mechanisms of this resistance and proposes a promising strategy to counteract.
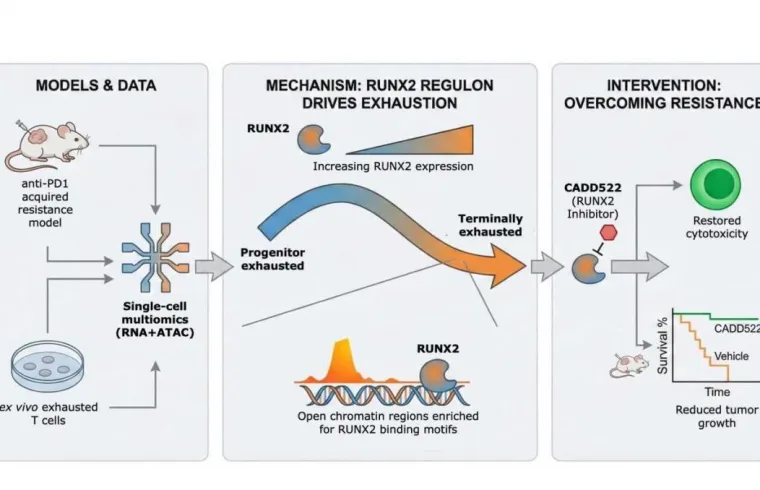
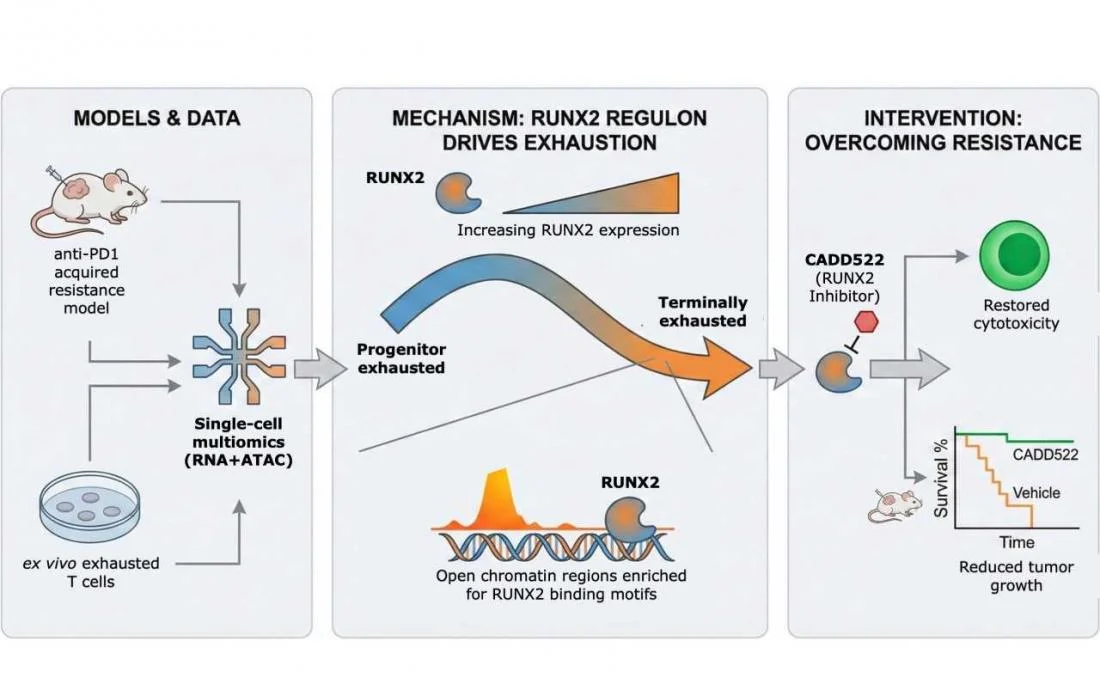
An interdisciplinary research team from National Taiwan University and National Taiwan University Hospital, led by Prof. Hsueh-Fen Juan, has identified RUNX2, a gene regulator not previously recognized for its role in immune regulation, as a key driver of immune cell exhaustion in liver cancer. The study demonstrates that inhibiting RUNX2 can restore immune cell function and suppress tumor growth, even after resistance to immunotherapy has developed. The findings were recently published in Molecular Cancer.
ICIs exert their therapeutic effects by lifting molecular “brakes” on CD8⁺ T cells, the immune system’s principal antitumor effectors. Although this immune reactivation can initially induce tumor regression, sustained immune stimulation may drive T cells into a dysfunctional state known as T-cell exhaustion, characterized by a progressive loss of their cancer-killing capacity.
Using advanced single-cell multiomics technologies, the researchers tracked the progression of T-cell exhaustion with unprecedented resolution. By simultaneously profiling gene expression and chromatin accessibility in individual T cells, they delineated the gradual transition from active immune responders to terminally exhausted states during prolonged anti-PD1 treatment. Analyses of human liver tumor samples further revealed that patients who failed to respond to immunotherapy harbored a significantly higher proportion of RUNX2-positive exhausted CD8⁺ T cells.
To determine whether RUNX2 actively drives immunotherapy resistance rather than merely serving as a marker of T-cell exhaustion, the team evaluated a small-molecule RUNX2 inhibitor in both cultured cells and mouse models of liver cancer that had developed resistant to immune checkpoint blockade. RUNX2 inhibition restored the cytotoxic function of exhausted T cells and significantly suppressed tumor growth, indicating that targeting this pathway may reinvigorate antitumor immunity even after immunotherapy failure.
Beyond identifying a novel therapeutic target, the study provides a broader conceptual framework for understanding immunotherapy resistance, not solely as a consequence of tumor escape mechanisms, but as a progressive reprogramming of immune cells themselves.
“Our findings identify RUNX2 as a promising target for next-generation immunotherapy,” said the study’s co-corresponding author, Hsueh-Fen Juan, Ph.D., a distinguished professor of life science at National Taiwan University.
“They suggest new opportunities for combination treatment strategies aimed at preventing or reversing immune exhaustion, as well as for developing biomarkers to identify patients at risk of immunotherapy resistance.”
As immunotherapy assumes an increasingly central role in cancer treatment, strategies that preserve immune cell function, such as targeting RUNX2, may enhance the durability and efficacy of these therapies in liver cancer and potentially across a broader spectrum of tumor types.