Reducing carbon dioxide (CO₂) concentration through electrochemical methods (eCO₂RR) in acidic conditions is an important strategy for producing valuable products while avoiding the formation of carbonate.
However, one major challenge is that in acidic media, the hydrogen evolution reaction (HER) tends to dominate because of the high availability of protons, making it harder for CO₂ reduction to occur efficiently.
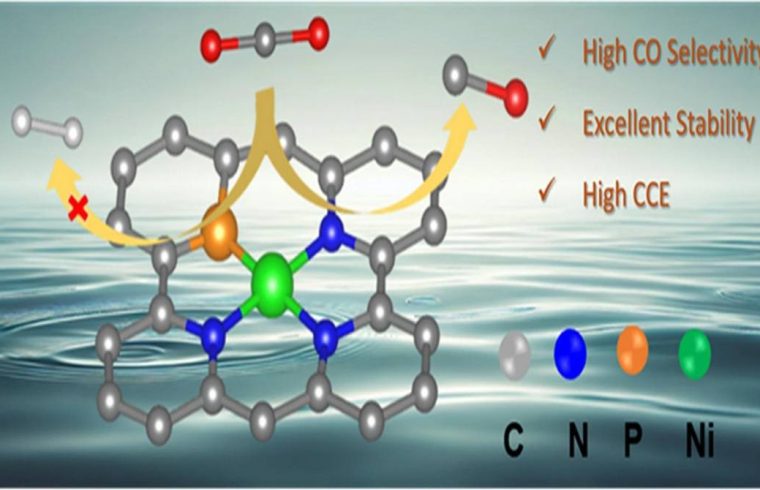
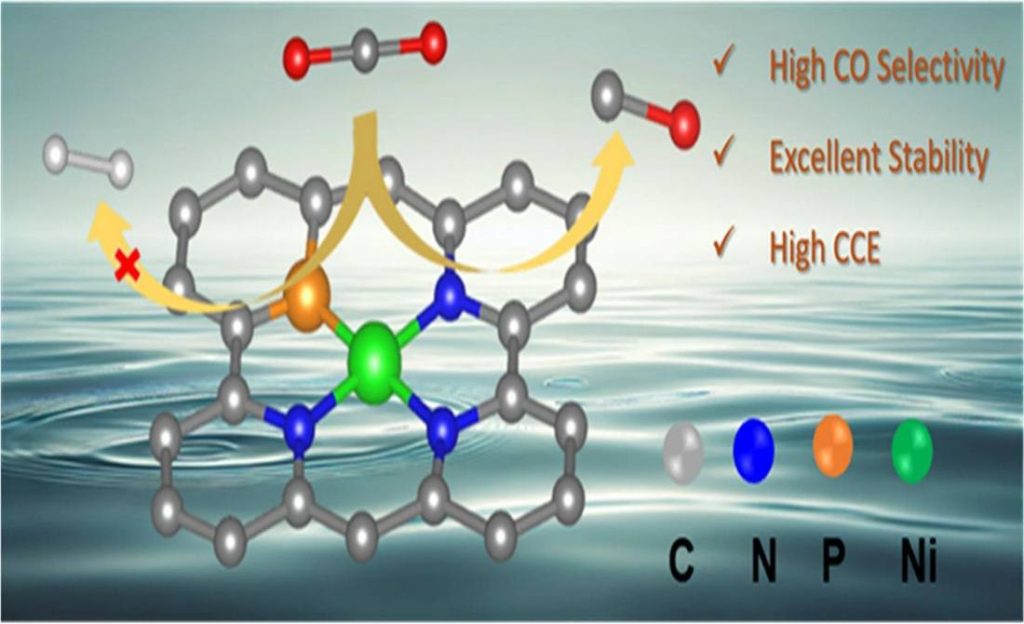
To address this issue, this study synthesized Ni–N3PC catalyst using an in-situ phosphatization method to modulate the electronic structure of the catalyst to suppress the competing HER and favor the CO₂ reduction reaction.
The Ni–N3PC catalyst showed excellent performance, with over 90% selectivity for producing carbon monoxide (CO) across a wide range of potentials. It also maintained high carbon efficiency and achieved a high partial current density for CO production, reaching –357.7 mA cm⁻².
In long-term tests, the catalyst remained stable for 100 hours, maintaining 85% CO selectivity at –100 mA cm⁻² without changing its structure. Further analysis using electrochemical impedance spectroscopy and turnover frequency studies of Ni–N3PC showed that it has lower charge transfer resistance and better intrinsic activity than Ni single atom catalyst.
The exceptional catalytic activity of Ni–N3PC arises from several synergistic effects. First, the in-situ phosphatization process modifies the electronic structure of the catalyst, which enhances its intrinsic activity. Additionally, the catalyst possesses a large surface area that boosts CO₂ adsorption capacity, ensuring a higher concentration of reactants at the active sites.
The XPS and XAS analyses confirm the presence of Ni–P and Ni–N bonds, while scanning transmission electron microscopy shows atomically dispersed Ni atoms on a carbon matrix.
“Computational studies supported the experimental findings, showing that the catalyst reduces the energy barrier for forming the key reaction intermediate (*COOH), thereby promoting CO generation. This research demonstrates a promising direction for designing efficient and stable catalysts for industrial CO₂ reduction,” said Prof. Li-Chyong Chen.