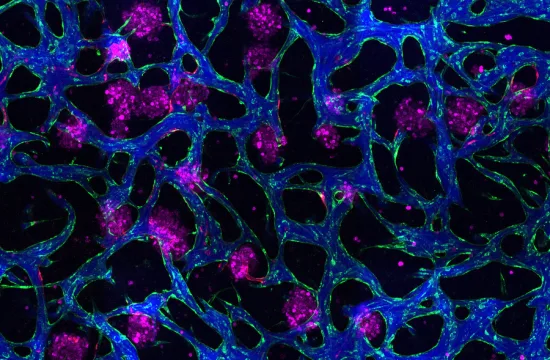
![Gene Therapy Makes Deaf Mice Hear 2 Researchers have designed an adeno-associated virus that delivers the Tmc gene into the hair cells of the inner ear, which restored hearing in genetically deaf mice. [Credit: V. Altounian/ Science Translational Medicine]](http://revoscience.com/en/wp-content/uploads/2015/07/getPart-300x225.jpg)
For the first time, gene therapy partially restored hearing to young mice who were born stone-deaf due to a human deafness gene mutation.
In a Science Translational Medicine study out this week, a group led by Harvard Medical School otologist Jeffrey Holt, Ph.D., reported they restored hearing to postnatal mice born deaf due to a gene that mutates in four to eight percent of children with inherited deafness: the TMC (transmembrance channel-like) 1 gene.
“We were optimistic this strategy might work, but when it actually did, it was one of those `eureka’ moments,” Holt told Drug Discovery & Development. “We used a `startle response’ assay. You can’t ask a mouse if he can hear something, but if you play a loud tone, a normal mouse will jump. Our TMC1 mutant mice are deaf; they don’t jump at all. They could care less about any sound. But when we took those mutant mice, and injected our gene therapy reagent into them, the mice started jumping. It was phenomenal.”
Columbia University Otolaryngology Chair Lawrence Lustig conducted the only other highly successful gene therapy preclinical study restoring hearing to deaf animals—if in his case, he corrected a gene that doesn’t cause congenital human deafness, just high-frequency age-related adult human deafness.
“This is an important work that significantly advances the field of gene therapy for genetic hearing loss on several fronts,” Lustig, uninvolved in the new study, told Drug Discovery & Development. “It validates and recapitulates prior studies of gene therapy rescue of function, but in a new and important model of deafness, and documents optimized viral and promoter subtypes for use in the ear.”
History

In 1958, a team of scientists characterized a line of deaf mice that lacked startle responses while lacking other disorders. There were many deaf mice, but usually the deafness accompanied other problems. These mice seemed purely deaf. They possessed normal hair cells—critical for translating sound, gravity, and head movements into electrical impulses—until two weeks post-birth. Then the hair cells died. In 1980, it was found deafness was linked to abnormal opening of hair cell membrane ion channels.
But it was not until a 2011 National Institute on Deafness and Other Communication Disorders (NIDCD) paper co-authored by Holt that key culprits were nailed down:
mutations in the TMC 1 and 2 genes. That paper showed, in vitro, that TMC1 and 2 are necessary for hair cell mechano-transduction. It showed that mice with TMC1 mutations were deaf, and mice with both TMC 1 and 2 mutations were deaf, and plagued with vestibular (balance) dysfunction.
Now Holt’s team has brought the work to animals.
“This really emerged out of the basic science,” he told Drug Discovery & Development. “We were interested in the TMC protein—TMC1 in particular– which we had found is part of a sensory transduction channel in ear cells to convert sound into electrical impulses transmitted to the brain. This is part of a 30-year search, itself pretty exciting. We found that protein carries many different mutations that cause deafness in humans. So we started thinking about how we could extend our work and go translational, develop a gene therapy for restoring auditory function.”
The gene therapy strategy
The strategy: “To take a viral vector called adeno-associated virus (AAV), which causes the common cold. We remove the viral gene, and put in the correct DNA sequence for TMC 1 (and/or TMC2). We introduce that vector into the ears of deaf mice that are a very good model for human genetic deafness….The vector carried the gene into the ear’s sensory cells. The ear cells knew what to do with the gene. They targeted it to the right spot in the cells to restore auditory function.”
Thirty days after injection, testing revealed the mice were taken from total deafness to “hearing something, if not to a complete recovery,” said Holt. “At the cellular level we get 100 percent recovery. And using our behavioral assay, we also see a complete recovery. But in the auditory brain stem (ABR) response, where we can measure responses of mice to different sound tones, we only see a partial recovery.”
The ABR involves placing electrodes on the scalp to measure electrical activity.
Inner vs outer hair cells
The main problem is the outer hair cells, he said. “We get very good recovery in sensory cells called inner hair cells, which we can transfect with the new gene at a rate of 80 to 90 percent. That is absolutely required to restore auditory function. But another class of cells, outer hair cells, are responsible for adjusting the sensitivity of the ear. For soft sounds they increase sensitivity; for loud sounds, they decrease it. We have only gotten five to ten percent transfection. We want to restore full function to those cells.”
His team is working on that. It could be a matter of changing the gene promoter involved, the vector, the site of injection.
His team’s other major project is longevity testing. They only tested deaf mice after a couple of months. They will extend that.
Grandma hearing sounds not sentences
Lustig told Drug Discovery & Development “the outer hair cell issue is real. We’ve had the same problem getting good transfection, and we don’t know why. It could be due to the fact that outer hair cells might be sealed off from inner hair cells, or have different receptors, making them more resistant to transfection.”
It is difficult to draw a human comparison, Lustig said. We can only tell whether mice can detect sounds “at particular volume and frequency.” After gene therapy, Holt’s deaf mice “can detect sounds at levels that are equal to normal mice. But the human equivalent of outer hair cell loss would likely be experienced as great sound detection, but not great word understanding. Volume is fine; clarity is an issue. Think of grandma hearing you talk, but unable to understand you. Of course, this is speculative.”
Well-executed research
Overall, Lustig said, the new study “is very well executed research, by a fantastic group. They clearly demonstrate hearing loss from a TMC1 gene defect can be partially corrected using virally mediated gene therapy. The work is similar to our prior work on the VGLUT-3 gene, but the TMC1 gene is a more significant type of hearing loss, occurring in more individuals.”
One other difference: Lustig’s VGLU3 mice only had an inner hair cell defect. The TCM1 gene affects both inner and outer hair cells.
“In addition to documenting robust inner hair cell transfection,” Lustig said, the Holt group “included electrophysiology documenting restoration of normal inner hair cell function.”
Their study’s “main limitation,” Lustig said, is that lack of outer hair cell transfection, and “failure to rescue otoacoustic emissions” so “outer hair cell function. This is a common problem that no one has been able to overcome. The real life affects in humans are unknown. But if we hope to make this practical in humans, I believe we will also need to get better outer hair cell transfection, with similar rescue of otoacoustic emissions.”
Holt agreed he’d like “the answer to those questions before clinical trial. We are also thinking about adapting this strategy for other forms of genetic deafness. Some 70 genes cause genetic deafness. It might be possible to adapt this strategy to those.”