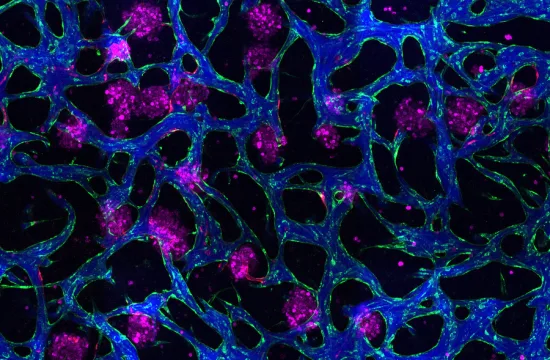
First identified in 2015, migrasomes are a newly discovered class of membrane-bound vesicles that form along the trails of migrating cells. Measuring less than 3 μm in diameter, these tiny structures serve as dynamic cargo carriers—transporting nucleic acids, proteins, organelles, and more.
Acting as both molecular messengers and functional hubs, migrasomes are now recognized as key players in organ development, immune modulation, and cancer metastasis, offering fresh perspectives on disease mechanisms and therapeutic innovation.
A comprehensive review by Dr. Chen Yang’s team at Peking University, titled “A Decade of Discovery: Tracing the Footmarks of Migrasomes, Unveiling the Footprints of Cells” (published in Science China Life Sciences), outlines the mechanisms behind migrasome formation, their diverse cargo, biological roles, and the cutting-edge technologies used to study them.
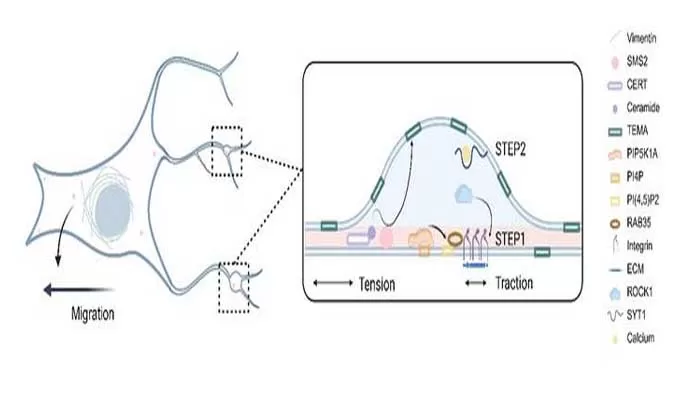
Biogenesis in Two Acts:
- Stage One: Sphingomyelin synthase 2 (SMS2) clusters at pseudopodia, marking the nascent sites of migrasome formation. This is followed by the assembly of a PI(4,5)P2-Rab35-integrin complex, which generates mechanical tension.
- Stage Two: Membrane swelling is driven by calcium-dependent SYT1, while TSPAN4-enriched microdomains (TEMAs) increase membrane stiffness, facilitating expansion and maturation.
Functional Versatility:
Once released, migrasomes engage in a wide array of physiological activities—supporting organogenesis, tissue repair, angiogenesis, hemostasis, and even viral transmission. Their dual nature in disease is particularly striking: they can aid recovery by clearing damaged mitochondria or delivering beneficial signals, yet also exacerbate pathology by transferring inflammatory cytokines or harmful molecules. Importantly, migrasomes are emerging as promising non-invasive biomarkers for early disease detection.
Recent innovations have significantly enhanced the efficiency of migrasome isolation and characterization. Live imaging breakthroughs now allow scientists to observe migrasome behavior in real time within living organisms. These advances are pushing the boundaries of organelle research and opening new avenues for precision medicine.
Despite remarkable progress, many mysteries remain. Future investigations will delve into how retraction fibers influence spatial cargo release and explore migrasomes’ specific roles in both healthy and diseased states. Unlocking their full potential will require interdisciplinary collaboration and continued innovation in multi-omics, advanced microscopy, and bioengineering.