Researchers at Kanazawa University in collaboration with teams from Toyama Prefectural University and BioSeeds Corporation report in ACS Applied Materials & Interfaces on the identification of a molecule with enhanced antiproliferative activity in cancer cells. The underlying biomolecular mechanism is the inhibition of an enzyme that is overproduced in several types of cancer.
As Vitamin D3 has important biological functions, including maintaining bone mineral density, which minimizes the risk of bone fracture. But vitamin D3 is also believed to have anticancer activity, as low vitamin D3 levels and the associated overproduction of an enzyme called CYP24 are linked to a poor prognosis for cancer patients.
Molecules that limit or inhibit the action of CYP24 and molecules that mimic the function of vitamin D3 are nowadays highly researched as potential antiproliferative agents for cancer treatment. But many of the inhibitors and D3 analogs synthesized so far have shown insufficient clinical response, as well as undesired side effects.
Now, Madhu Biyani from Kanazawa University and colleagues have identified a DNA-derived molecule that binds to and inhibits the function of CYP24 and shows promising antiproliferative activity. The research team also provides detailed insights into the relevant molecular processes at play.
The scientists screened a large number of DNA aptamers — pieces of single-stranded DNA with particular three-dimensional structures that can bind to specific target molecules and have a functional effect upon binding. They looked for DNA aptamers that bind to CYP24 but not to the similar enzyme CYP271B, which is responsible for the synthesis of vitamin D3.
An initial longlist of 18 aptamer candidates was reduced to 11 representatives with specific molecular structures. The researchers checked the CYP24 inhibition activity of the 11 representative aptamers in vitro. Four candidates resulting in the inhibition of CYP24 but not in the inhibition of CYP27B1 remained, of which one (Apt-7) was retained for further study.
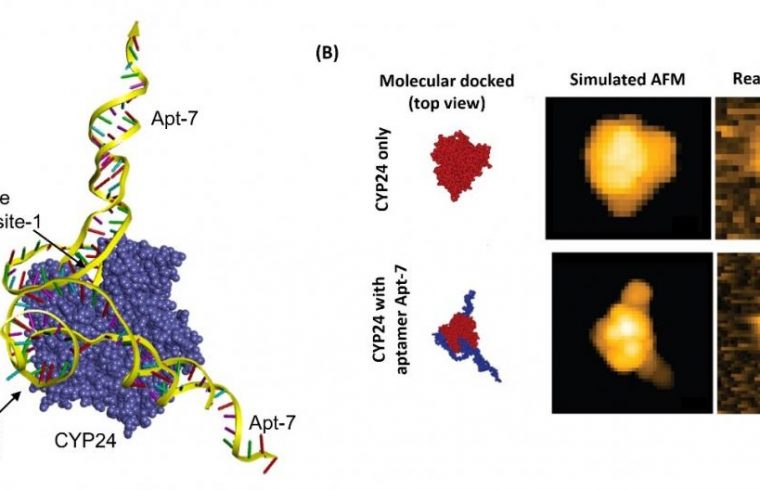
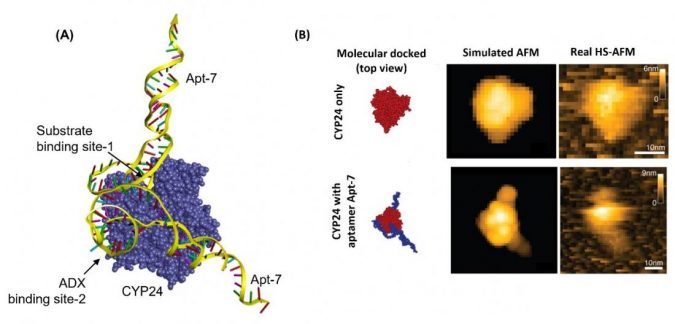
Biyani and colleagues performed simulations of Apt-7 binding to CYP24. A molecular docking scenario was obtained, which they checked experimentally by comparing the behavior of a mixture of vitamin D3 and CYP24 with and without Apt-7.
The simulations and the experiments showed that Apt-7 results in the inhibition of CYP24 activity and that what happens is that the aptamer likely interferes with the enzyme’s active site. The researchers also performed high-speed atomic force microscopy on the binding of CYP24 and Apt-7 in real-time, confirming the molecular docking scenario obtained from simulations.
In addition, the research team studied the effect of Apt-7 at the cellular level by introducing the molecule to cancer cells. They observed significant CYP24 inhibition for a cancer cell line known to overexpress the CYP24 enzyme, thus showing antiproliferative activity.
Quoting Biyani and colleagues, these findings “clearly characterized and proposed that a DNA aptamer-based molecule could be a promising lead candidate for anticancer therapy.