Scientists have discovered a sophisticated mechanism by which Salmonella bacteria hijack a mitochondrial metabolite transporter to survive inside host cells.
Their findings, published in Nature Communications, reveal how the pathogen reduces oxidative stress to evade the host defense system. This discovery paves the way for potential host-directed antimicrobial therapies.
Traditionally, the immune system fights intracellular bacteria like Salmonella by trapping them in membrane-bound compartments called vacuoles. Here, the host creates reactive oxygen species (ROS), which are toxic chemicals designed to kill the invaders.
However, Salmonella is resilient and frequently manages to survive and replicate within these hostile environments. Until now, the specific mechanisms enabling the bacteria to neutralize these oxidative defenses remained partially understood.
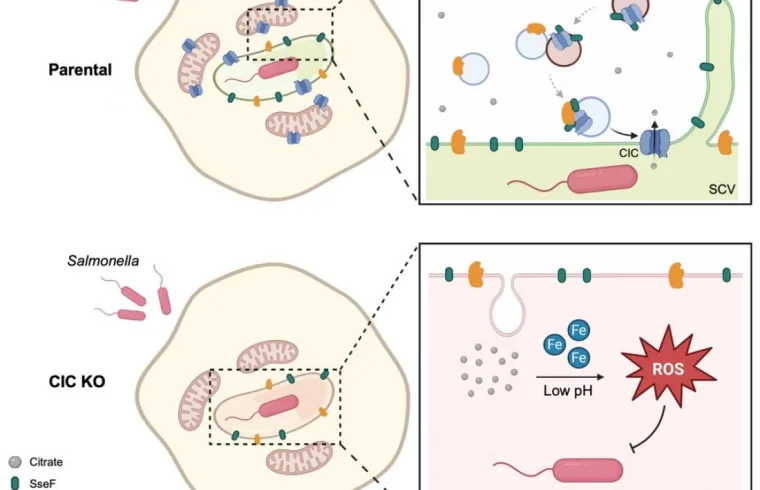
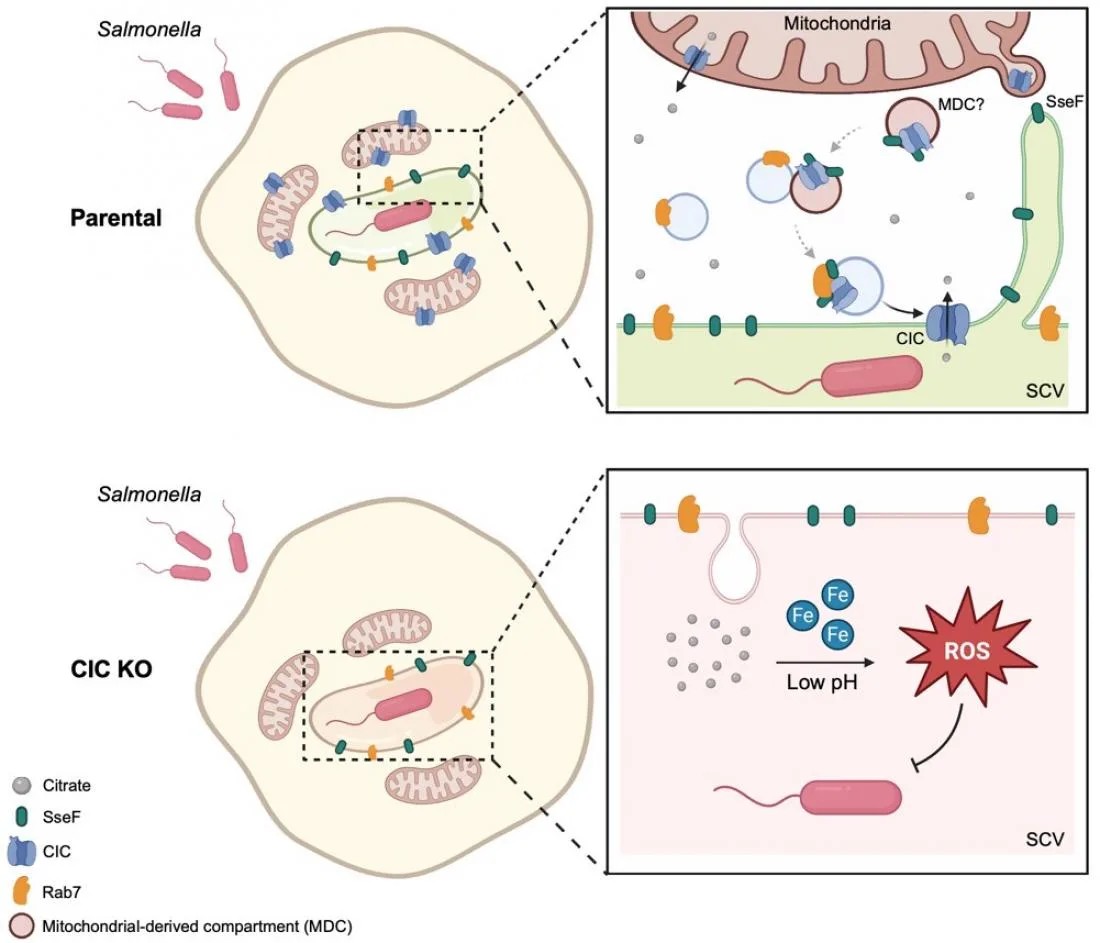
In this study, the research team found that Salmonella enterica serovar Typhimurium actively recruits a host protein called the mitochondrial citrate carrier (CIC) to the vacuole where it hides. The bacteria repurpose it to export citrate out of the vacuole. This efflux of citrate is critical for maintaining redox homeostasis and neutralizing toxic ROS buildup.
“We discovered that Salmonella doesn’t just endure the host’s defenses; it actively re-engineers the host cell’s resources to create a safer environment for itself,” said Professor Shu-Jung Chang from the Graduate Institute of Microbiology at National Taiwan University and corresponding author of the study.
“By hijacking the CIC, the bacteria effectively detoxify their surroundings, allowing them to multiply unchecked.”
The researchers identified a specific bacterial protein known as SseF that acts as the key to this process. SseF interacts with the host’s CIC and the protein RAB7 to physically drag the mitochondrial transporter onto the bacterial vacuole.
To test the therapeutic potential, the team used pharmacological inhibitors to block the CIC transporter. The results were promising. When CIC was inhibited, Salmonella lost the ability to control oxidative stress and became significantly more vulnerable to immune attacks.
These findings suggest that targeting this hijacked host pathway could sensitize bacteria to the body’s own immune pressure. This host-directed approach offers a new strategy to combat the global threat of antibiotic resistance.