NTU Singapore-– Scientists at Nanyang Technological University have discovered a new molecule which can join together chains of amino acids – the building blocks of protein.
Only three other known molecules have been discovered to be able to perform this function, which is an important process in the development of new drugs. A key difference is that the new molecule is able to do the same process 10,000 times faster than the other three and “cleanly” without leaving any residue behind.
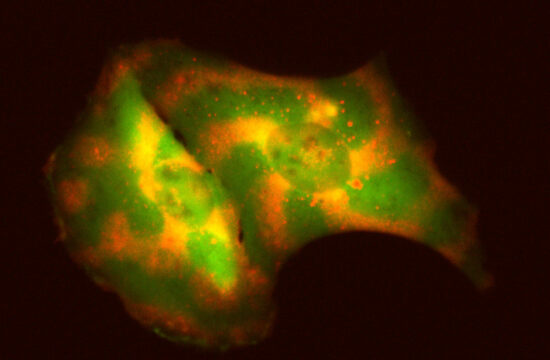
This new molecule, which is a type of catalyst or enzyme, was derived from a common medicinal plant found in Singapore and Southeast Asia. Scientifically known as Clitoria ternatea or the Blue Butterfly Pea, this plant’s blue flowers are commonly used to make food colouring such as those used in Pulut Inti, a Malay glutinous rice delicacy. The plant is also commonly used as a traditional herb to enhance memory, as well as an anti-depressant and anti-stress agent.
Named Butelase-1 after the plant’s Malay name Bunga Telang, the new enzyme acts as a ligase, joining longer chains of amino acids known as proteins or peptides (which are smaller in size than proteins) together.
Professor James Tam from NTU’s School of Biological Sciences, the lead scientist of this project, said that the properties of the new molecule make it a very useful tool in protein biotechnology and the development of new peptide and protein therapeutics, including anti-cancer agents.
“While we already have the tools to cut peptides easily, joining them together is much harder, as the most commonly used ligase usually leaves residues behind, which may affect the efficacy of the final product,” said the director of NTU’s Drug Discovery Centre.
“The ability to cut and join bits and pieces of peptides fast, easily and cleanly will speed up our search for novel drugs and treatments.”
Prof Tam’s breakthrough discovery was first published last month in Nature Chemical Biology, a top international scientific journal. The significance of the finding resulted in no less than four independent commentaries so far published by Nature Publishing Group, Structural Biology Knowledgebase, and Science Business eXchange – all top scientific publishers.
Ligase such as Butelase-1 is the reason why plants are able to join together end-to-end to create circular chains of amino acids, commonly known as macrocycles. Such circular chains make plants more resilient to harsh weather such as extreme cold or heat.
These circular proteins were rarely found before the 1990s, and their biosynthesis had remained a mystery until the NTU team published their work. Butelase-1 was found to be responsible for this backbone cyclization – the circular joining of a protein chain.
The NTU researchers believe that their discovery of Butelase-1 could potentially change the landscape of how proteins are studied and manipulated in the development and design of new drugs.
This project, which took about a year to complete, is part of a bigger herbalomics research programme led by Prof Tam, which received a $10 million grant under the National Research Foundation’s (NRF) Competitive Research Programme (CRP) in 2012.
The herbalomics programme aims to do a system-wide analysis of active ingredients in functional food and herbal medicine. Key goals of the programme include finding novel approaches to ensure the safety, quality and efficacy of plant-based products.
This research, which has impact on biomedical research and applications, is part of Future Healthcare, one of NTU’s Five Peaks of Excellence which are interdisciplinary areas of research that the university focuses on. The other four peaks are Sustainable Earth, Innovation Asia, New Media, and the Best of East and West.