Thousands of people enjoy fireworks with colors whenever its the time of celebration and these, the red, orange, yellow, green, blue and purple colors exploding in the night sky during a fireworks display are created by the use of metal salts.
In chemistry, a salt is defined as an ionic compound that is formed from the reaction of an acid and a base. Salts are electrically neutral compounds that are composed of both positively charged ions and negatively charged ions. For example, familiar table salt consists of both positive sodium ions (Na+) and negative chloride ions (Cl–).
Metal salts that are commonly used in firework displays include: strontium carbonate (red fireworks), calcium chloride (orange fireworks), sodium nitrate (yellow fireworks), barium chloride (green fireworks) and copper chloride (blue fireworks). Purple fireworks are typically produced by use of a mixture of strontium (red) and copper (blue) compounds.
The metal salts are packed into a firework as pea to plum sized pellets called stars. After a firework is ignited, a lift charge propels the firework into the sky while a fuse slowly burns into the interior of the firework shell. As the fuse reaches the core of the firework, it explodes igniting the stars that contain the metal salts.
When metal salts ignite, they emit light. The heat from the ignition causes an influx of energy into the metal atoms. As heat gets absorbed by the metal atoms, electrons that are circling around the lower orbitals of the atomic nucleus excite and jump to a higher energy level. As the heat dissipates, the electrons fall back to their lower energy level or ground state and release the extra energy in the form of light.
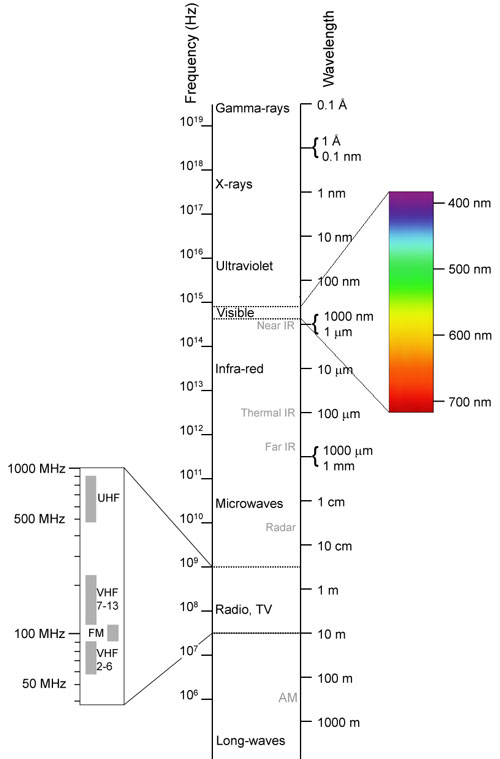
Light travels in waves. Different metal atoms produce different colors of light when excited because the light that they emit is traveling at different wavelengths. The wavelengths of light produced by metals vary in accordance with the amount of energy that is released by the unique arrangement of electrons in the atom as it falls from an excited state back to a ground state.
When metal salts emit short wavelengths of visible light in the range of 400 to 500 nanometers, they produce violet and blue colors. When metal salts emit longer wavelengths of visible light in the range of 600 to 700 nanometers, they produce orange and red colors. Yellow and green colors are produced by metal salts that emit visible light at intermediate wavelengths (500 to 600 nanometers) along the electromagnetic spectrum.