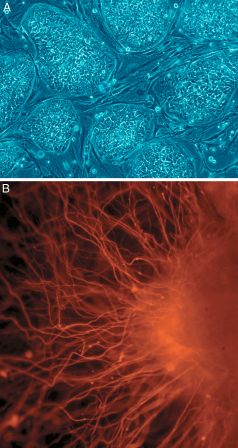
Upping the stakes in the enduring competition between pluripotent/embryonic stem cells made via the iPSC method, and via cloning, a Harvard University group has reported dramatically improving the efficiency of cloning.
For the first time, quality blastocysts have been derived from a full 14 percent of adult cells via cloning (somatic cell nuclear transfer: SCNT), and embryonic stem (ES) cells were created from an unprecedented 50 percent of those.
“This 50 percent success rate is as high as one can get even using in vitro fertilization (IVF) embryos,” Harvard stem cell biologist Yi Zhang, Ph.D., senior author, told Drug Discovery & Development.
Robert Lanza, Ph.D., Ocata Therapeutics CSO, told Drug Discovery & Development: “This is an important discovery. Considering the extreme difficulty in obtaining human eggs, this study represents a major advance in the ability to use SCNT, not only for drug discovery, but for the potential treatment of human diseases, such as mitochondrial-related disorders.” Lanza was uninvolved with the study.
From mouse to man
In cellular cloning (again, SCNT), the nucleus of an adult cell—which contains all of our DNA except our mitochondrial DNA—is placed in an enucleated egg cell. Once ignited by chemicals or a zap of electricity, the egg cell is tricked into thinking the new nucleus is sperm-related, and turns back the clock on the old DNA, returning it to an embryonic state.
ES cells that are immunologically identical to their adult donor can then be culled.
[pullquote]For the first time, quality blastocysts have been derived from a full 14 percent of adult cells via cloning (somatic cell nuclear transfer: SCNT), and embryonic stem (ES) cells were created from an unprecedented 50 percent of those.[/pullquote]
However, efficiency has been low. Last year, for a Cell paper, Zhang’s crew compared epigenomic and transcriptomic data on SCNT and IVF mouse embryos, and decided the low efficiency rate may be due to epigenetic methylation, which was keeping the adult donor cells switched into an adult state. So they injected into cloned mouse cells RNA encoding a histone demethylase, or enzyme, that blocks the kind of methylation they identified as problematic.
Specifically, they injected RNA encoded for the H3K9me3 demethylase, Kdm4d. The methylation tag was blocked, and efficiency greatly improved.
Success rate better than normal IVF ES cells
This year, the team tried it again, on human cells. It worked again. No SCNT embryos without the added RNA made it to the expanded blastocyst stage. With the treatment, there was a 14 percent success rate.
“The quality of the expanded blastocyst affects the rate of ES cell derivation,” Zhang told Drug Discovery & Development. Even during IVF, where normal ES cells are culled, “not all the expanded blastocysts generated can successfully generate ES cell lines. In our study, eight SCNT embryos reached the expanded blastocyst stage. From this, we successfully derived four lines of ES cells that survived a long time in culture.” That success rate bests that of the rate achieved by stem cell scientists culling ES cells from spare IVF clinic embryos.
Given that Zhang’s ES cells, like all ES cells from cloning or SCNT, come from adult cells, this theoretically could make SCNT ES cells not just less controversial than normal ES cells, but more plentiful.
Zhang believes his cells are more physiologic than pluripotent cells made from adult cells via the induced pluripotent stem cell (iPSC) method. In that method, four genes are tweaked to bring adult cells back to am embryonic state. But those embryonic cells are not totipotent, that is, able to create all cells of the body, including the placenta.
“For the mouse work that we published last year in Cell, we demonstrated that SCNT reprogramming can generate both the embryos and placenta,” he said. “Since it is illegal to implant human SCNT embryos, we are not allowed to test whether the human SCNT embryos can also contribute to placenta. However, theoretically, people believe reprogramming through SCNT can generate totipotent cells.”
Another reason he believes SCNT ES cells are more physiologic than iPSC cells: “IPSC reprogramming is totally artificial as it does not generate blastocysts from which the pluripotent ESCs are derived,” Zhang said. “SCNT reprogramming generates blastocysts, which are the source of real ES cells.”
Furthermore, Zhang said: “Many studies indicate that iPSCs possess epigenetic memory of their donor somatic cells unless it cultured in vitro for a long time. Yet culture in vitro for a long time can be a source of mutation.”
Finally, he said, since mitochondria are passed to offspring via eggs, SCNT cells possess the mitochondria of the donor egg. So when the adult nucleus donor has a mitochondrial disease, generating stem cells using the adult’s DNA and healthy eggs may mitigate the mitochondrial problems. This is not true of iPSC-derived pluripotent cells.
“Patient somatic cell-derived iPSCs cannot fix mitochondria DNA because problems in the iPSC mitochondria DNA will be the same as the patient mitochondria DNA,” he said. “On the other hand, the mitochondria DNA of SCNT derived ESCs are derived from the healthy egg donor, not from the patient.”
Regarding whether SCNT ES cells are more “physiologic” than iPSCs, Lanza told Drug Discovery & Development: “The evidence suggests they may be, but more work needs to be done to know for sure.”